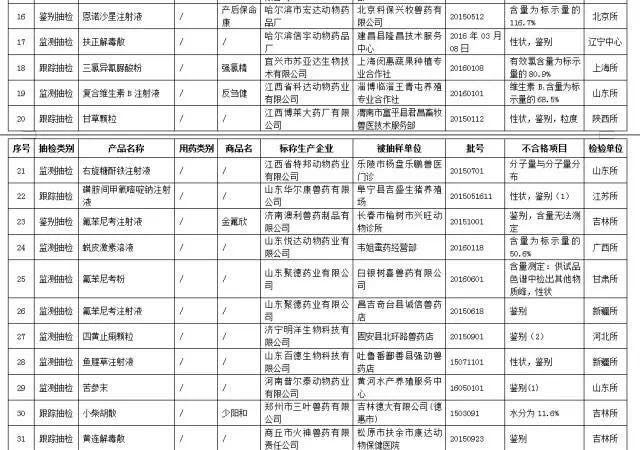
The ministry of agriculture has requested that the two companies focus on the monitoring of the two enterprises and punish enterprises that involve 19 batches of substandard products. It is understood that many of these 144 unqualified products are well-known brands in the industry.
In recent days, the ministry of agriculture announced the quality supervision and sampling of veterinary drugs in the fourth period of 2016. It is understood that 144 batches of unqualified veterinary drugs and biological products were investigated in the third quarter.
Overall, in the third quarter of 2016, a total of 179 batches of animal biological products were inspected, 178 batches qualified, unqualified 1 batch (annex 1), and the qualified rate was 99.4 percent.
A total of 3,622 batches of veterinary drugs and Chinese veterinary drugs were completed, and 34,79 approved batches were qualified. 143 batches were not qualified (annex 2, annex 3), and the qualified rate was 96.0%.
Among them, veterinary drug monitoring and sampling 2580 batches, qualified 2478 batches, qualified rate 96.0%; Veterinary drug tracking 505 batches, qualified 482 batches, qualified rate 95.4%; Veterinary drug targeted sampling 76 batches, qualified 76 batches, qualified rate 100.0%; Veterinary drug identification 461 batches, qualified 443 batches, qualified rate of 96.1%.
In addition, the ministry of agriculture also according to the key monitoring enterprise division principle, decides the following two enterprises as the key monitoring enterprises. (1) fuyang branch of Shanghai shenguang animal health products co., LTD. (2 batches below 50%); (2) guangxi hepu hongxiang animal pharmaceutical co., LTD. (2 batches below 50%).
Report of the ministry of agriculture on the quality supervision of veterinary drug quality in the fourth period of 2016
The animal husbandry and veterinary services (bureaus, commissions and offices) of the provinces, autonomous regions and municipalities directly under the central government (bureaus, commissions, offices), xinjiang production and construction corps animal husbandry and veterinary services:
The quality of veterinary drug quality supervision in the third quarter of 2016 is reported as follows.
1. Basic information
(1) animal biopsies
In the third quarter of 2016, a total of 179 batches of animal biological products were inspected, 178 batches qualified, unqualified 1 batch (annex 1), and the qualified rate was 99.4 percent.
(2) sampling of veterinary drugs and veterinary drugs
In the third quarter of 2016 has accomplished in the veterinary chemicals, veterinary drug supervision and sampling, 3622, 3479 batches of qualified and unqualified batch (annex 2 and annex 3), 143 qualified rate 96.0%, than the second quarter of 2016 (97.3%) fell 1.3%, (95.3%) than the same period in 2015 increased by 95.3%. Among them, veterinary drug monitoring and sampling 2580 batches, qualified 2478 batches, qualified rate 96.0%; Veterinary drug tracking 505 batches, qualified 482 batches, qualified rate 95.4%; Veterinary drug targeted sampling 76 batches, qualified 76 batches, qualified rate 100.0%; Veterinary drug identification 461 batches, qualified 443 batches, qualified rate of 96.1%.
From the sampling inspection process, 645 batches were detected in the production section, 632 batches qualified, and the qualified rate was 98.0%, a decrease of 0.5 percentage points from the second quarter of 2016 (98.5%). In the second quarter of 2016, the total of 2,450 batches and 2,339 approved batches was up to 95.5%, which was 1.4 percentage points lower than the second quarter of 2016 (96.9%). With 527 batches and 508 batches, the qualified rate was 96.4 percent, down 1.3 percentage points from the second quarter of 2016 (97.7 percent).
In terms of product category, 1439 batches of pharmaceutical products were detected and 1384 qualified. The qualified rate was 96.2%, which was 1.7 percentage points lower than the second quarter of 2016 (97.9%). In total, 1271 batches of antibiotic products were detected and 1236 approved. The qualified rate was 97.3%, down 0.3 percentage points from the second quarter of 2016 (97.6%). A total of 883 batches of TCM products were collected and 830 approved. The qualified rate was 94.0%, 1.7 percentage points lower than the second quarter of 2016 (95.7%). 29 batches of other products were detected, 29 batches qualified and 100.0% of qualified rate.
In the third quarter of 2016, the veterinary drug test product related information, please go to the "China veterinary medicine information network" "national veterinary medicine basic information inquiry system" inspection.
Ii. Key monitoring enterprises
According to the key monitoring enterprise division principle, the following two enterprises are judged to be the key monitoring enterprises.
(1) fuyang branch of Shanghai shenguang animal health products co., LTD. (2 batches below 50%);
(2) guangxi hepu hongxiang animal pharmaceutical co., LTD. (2 batches below 50%).
3. Main problems existing
In the third quarter of 2016, the non-conformance of veterinary drug testing was not qualified, and the content of some products was low, the content of individual products was even 0, and the content of individual products was not determined.
4. Work requirements
(1) implement the provisions for heavier punishment
All localities shall, in accordance with the provisions of the ministry of agriculture's public notice no. 2071, comply with the provisions of the provisions of the provisions of the provisions of the ministry of agriculture (annex 4), and shall be given a heavier punishment according to law. If the license for the cancellation of veterinary drugs is revoked, the license for production of veterinary drugs shall be revoked and the penalty shall be imposed. To identify test unqualified, the inspection agency to further test, confirm whether there is a change agent formula, illegal adding other ingredient, such as illegal behavior, to provide technical support for the administrative penalties. Provincial veterinary administrative departments should be timely to investigate results or investigation, according to the general office of the ministry of agriculture on further strengthening veterinary drugs illegal case investigation and information work to submit the notice (agricultural medicine [2016] no. 16) requirements, transfer the relevant case materials, investigation report and submitted to the ministry's. My department will report the work situation in due course.
(2) organize and carry out inspection activities
Around to notify JiaLie veterinary medicine, to concentrate on to investigate and punish, shall be ordered to recall destroyed JiaLie veterinary medicine production enterprises, the relevant illegal enterprises should implement the flight check, investigate and punish the illegal ACTS in accordance with the law. Among them, the content of identification, the unqualified products and does not belong to any of the circumstances set shall be given a heavier punishment, to order the companies to stop the production of the product, the enterprise is located and veterinary administrative department at the provincial level of qualified audit corrective, before resuming production.
(iii) improving the quality information of veterinary drugs
Across to the sampling results timely information about veterinary medicine management enterprises within their respective jurisdictions, animal farm, village, households) and other relevant units, prevent farmers buy by mistake, the misuse of JiaLie veterinary drugs, breed tache medication safety.
(4) continue to strengthen daily supervision
We should strengthen the daily supervision of the production and operation of veterinary drugs in the region, strengthen the follow-up supervision measures of veterinary drug GMP and veterinary drug GSP, and deal with the illegal ACTS found in the regulation in a timely manner.
The ministry of agriculture, December 8, 2016
Jiangxi province national plan unqualified product
|


